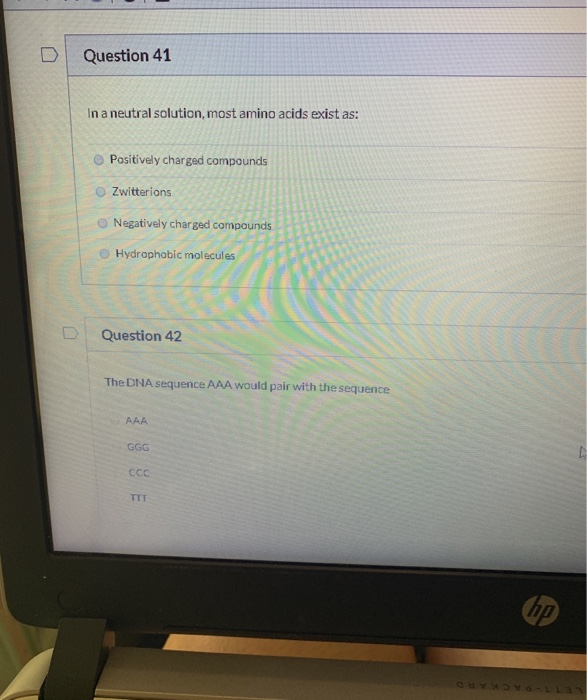
In a Neutral Solution Most Amino Acids Exist as
Normally an amino acid produces a nearly neutral solution since the acid group and the basic amine group on the root amino acid neutralize each other in the zwitterion. Chemistry questions and answers.
Amino Acids And Peptides Ppt Download
Depending on the pH there are two other forms an anion and a cation.
. O pH pKa Equal amounts of protonated and deprotonated species exist if pH is LESS than the pKa of a particular group that. DI Question 41 In a neutral solution most amino acids exist as. Because of the charges.
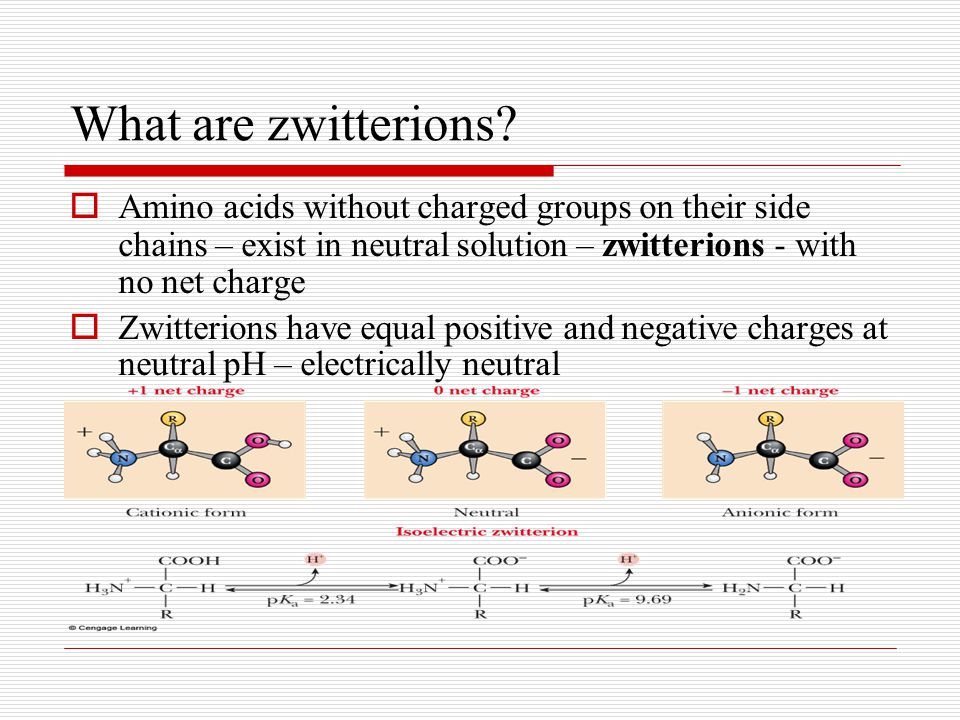
Most amino acids exist as zwitterions dipolar ions at pH 7. In a neutral solution most amino acids exist as. The particular pH at which a given amino acid exists in solution as a zwitterion is called the isoelectric point pI.
Note that all amino acids are at one point electrically neutral at some pH value. In a neutral solution most amino acids exist as. In aqueous solution amino acids exist in two forms.
This parallels the behavior of a diprotic acid. Justify your answers using Equation 164. The structure of a zwitterion is shown below.
In neutral solution solution amino acid present as ZW View the full answer Transcribed image text. Their side chains contain nitrogen and resemble ammonia which is a base. The amino acids generally form diprotic or triprotic systems in aqueous solution.
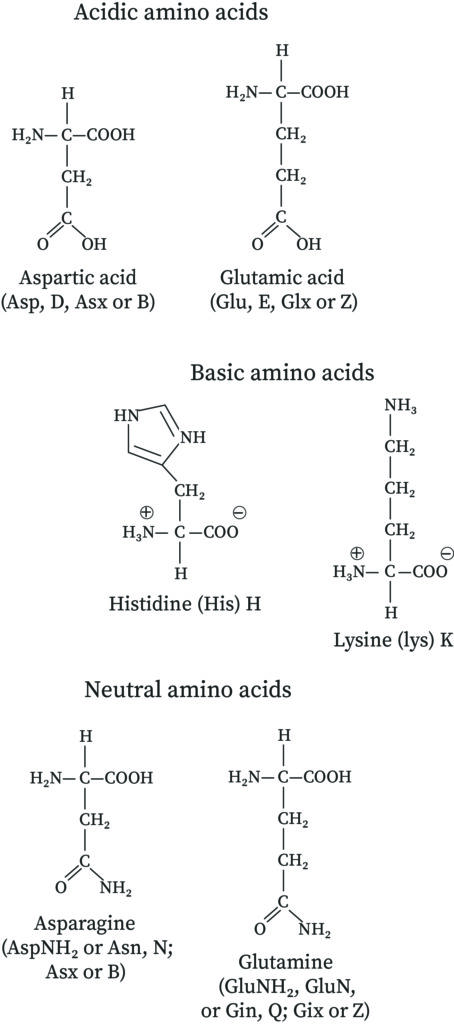
If the amino acid structure contains two acid groups and one amine group there is a net acid. Amino Acids as Acids Bases and Buffers. Asparagine and glutamine are neutral amino acids.
A the overall molecule is positively charged B the overall molecule is negatively charged C the overall molecule is electrically neutral D None of the above. The standard amino acids are therefore classified on the basis of these R groups. Negatively charged compounds D.
Their pKas are high enough that they tend to bind protons gaining a. The remaining amino acids have. The R group represents the side chain of different amino acids21 jan.
23 rows If the side chain contains an acid functional group the whole amino acid produces an acidic solution. There are three amino acids that have basic side chains at neutral pH. In a neutral solution most amino acids exist as.
Amino acids that contain only the α amino and α carboxyl groups which act as Brønsted-Lowry acid-base conjugate pairs somewhere within the normal aqueous pH range meaning that the p K a of the acidic form of the pair lies between 0 and 14 effectively form a. In the solid state amino acids exist as dipolar ions called zwitterions. There are certain amino acids that are capable of being synthesized in the human body which are referred to as non-essential amino acids.
These are arginine Arg lysine Lys and histidine His. The neutral zwitterion is the usual form amino acids exist in solution. When monoamino-monocarboxylic amino acids exist in a buffer solution of pka 7 what is the overall molecule charge.
Most of these amino acids differ only in the nature of the R substituent. A positively charged compounds. In aqueous solutions amino acids mostly exist as H3 NCHRCOOzwitter ion.
Amino acids can also be further categorized into Non-essential. In a neutral solution most amino acids exist as. Two amino acids that contain sulfur atoms are.
They have an amino group NH3 which is positive and a carboxyl group COO- which is negative. In this situation the acidic and basic groups on the amino acids will be ionized. Are all amino acids zwitterions.
Using glycine as an example and given that the pKa of the carboxyl group is 23 and that of the ammonium group is 96 predict the predominant form of the molecule at pH 1 7 and 12. C negatively charged compounds. An amino acid has this ability because at a certain pH value different for each amino acid nearly all the amino acid molecules exist as zwitterions.
A very small fraction of amino acid molecules will be neutral with a deprotonated amino groum and a protonated carboxylic acid group. The amino acids whose side chains are always neutral have isoelectric points ranging from 50 to 65. However the overwhelming majority of molecules will be in a zwitterion tautomer with a positive charge on.
If acid is added to a solution containing the zwitterion the carboxylate group captures a hydrogen H ion and the amino acid becomes positively charged. Positively charged compounds B. Amino acids with nonpolar substituents are said to be hydrophobic water-hating.
O Positively charged compounds O Zwitterions O Negatively charged compounds Hydrophobic molecules Question 42 The DNA sequence AAA would pair with the sequence CCc ip. Since proteins are biological molecules they are usually found in a neutral solution that is buffered at pH 70 to 74. In a neutral solution most amino acids exist as.
Some of the non-essential amino acids are aspartate alanine asparagine cysteine glycine arginine. At neutral pH amino acids exist as dipolar ions. In a neutral solution most amino acids exist as A Positively charged B from NURSING 231 at Saint Vincents College.
With two dissociation steps controlled by two acidity constants K 1 and K 2. This pH isoelectric point pI. Amino acids are the most well-known zwitterions.
At its pI the positive and negative charges on the amino acid balance and the molecule as a whole is electrically neutral. Amino acids with polar R groups that form hydrogen bonds to water are classified as hydrophilic water-loving.
Solved Di Question 41 In A Neutral Solution Most Amino Chegg Com
No comments for "In a Neutral Solution Most Amino Acids Exist as"
Post a Comment